Viral Nucleic Acid Detection Overall Solution (Part 2)
Prev Article:Viral Nucleic Acid Detection Overall Solution (Part 1)
1. Product Introduction
1.1 Instrument
The Maverick qPCR system (MQ4) is developed by Anitoa based on the "core chip". All specifications and models of the MQ4 ultra-portable qPCR instrument have passed CE-IVD certification and FDA registration.
1.1.1 Product Features
(1) User-friendly
10-inch screen Integrated touch screen operation. No external computer is needed. User-friendly operating system, user-friendly design, easy to use.
(2) Stable and Reliable
Using fixed, non-moving optical components, long-term use does not require expensive and cumbersome regular calibration and maintenance. Compatible with mainstream standardized consumables on the market. The stable, efficient and convenient output of test results improves the accuracy and timeliness of making relevant decisions.
(3) Lightweight and Portable
The instrument is small and portable (247*188*133mm), weighs only 2.6kg, and is convenient to move, especially suitable for places with limited laboratory testing space.
(4) Multiple Choice
Support 2/4 channel fluorescence channels (more channels can be customized), applicable to most of the current dyes, no cross-interference between channels, no need for regular calibration and maintenance.
(5) Flexible Combination
Multiple instruments can be flexibly combined to achieve 16, 32, 48, or more detection requirements, which fully meets the on-site detection, which can be detected immediately.

1.1.2 Application Scenarios
(1) Rapid detection of small-batch samples in the emergency department, fever clinic, ICU, etc.
(2) Rapid detection of customs and airport port quarantine.
(3) Rapid detection of epidemic prevention and control by the CDC in various regions.
(4) The popularity of nucleic acid testing in primary medical units.
(5) Daily supervision and inspection by the public health department.
(6) Emergency on-site quarantine (mobile shelter, mobile inspection vehicle or biological safety cabinet, etc.).
(7) Food safety testing.
1.2 Compatible Novel Coronavirus Reagent
The Anitoa COVID-19 testing solution is mainly used for the qualitative detection of novel Coronavirus infection suspected cases of pneumonia, suspected clusters of cases, and another novel Coronavirus (SARS-COV-2) RdRp, N, and S genes in nasopharyngeal swab samples requiring the diagnosis or differential diagnosis of Novel Coronavirus infection.
1.2.1 Product Features
(1) Convenient transportation: using a freeze-dried reagent, no cold chain transportation, convenient storage;
(2) Simple reagent: Nucleic acid extraction is not required, the sample can be directly added to the detection reagent, and then the instrument can be used for amplification detection.
(3) Fast detection: 16 samples can be tested within 40min.
(4) High sensitivity: 500-800copies/mL ;
(5) Multi-index detection: multiple detections, a single tube can simultaneously detect novel coronavirus (RdRp gene and N gene), Delta, and internal reference genes.
2. Proprietary Core Technology
Anitoa's "new ultra-low-light CMOS image sensor (CIS) chip" was developed by Dr. Zhimin Ding, founder and chief scientist of Anitoa, for medical instruments in Silicon Valley, USA. This ULS24 bio-optical sensor is an ultra-sensitive image sensor IC (integrated circuit), manufactured with mature and low-cost semiconductor technology, and enhanced by Anitoa's intelligent dark current management algorithm. Taking pictures simultaneously of all 576 pixels on a single chip can achieve higher data consistency than ordinary photoelectric sensors.
2.1 Platform Comparison
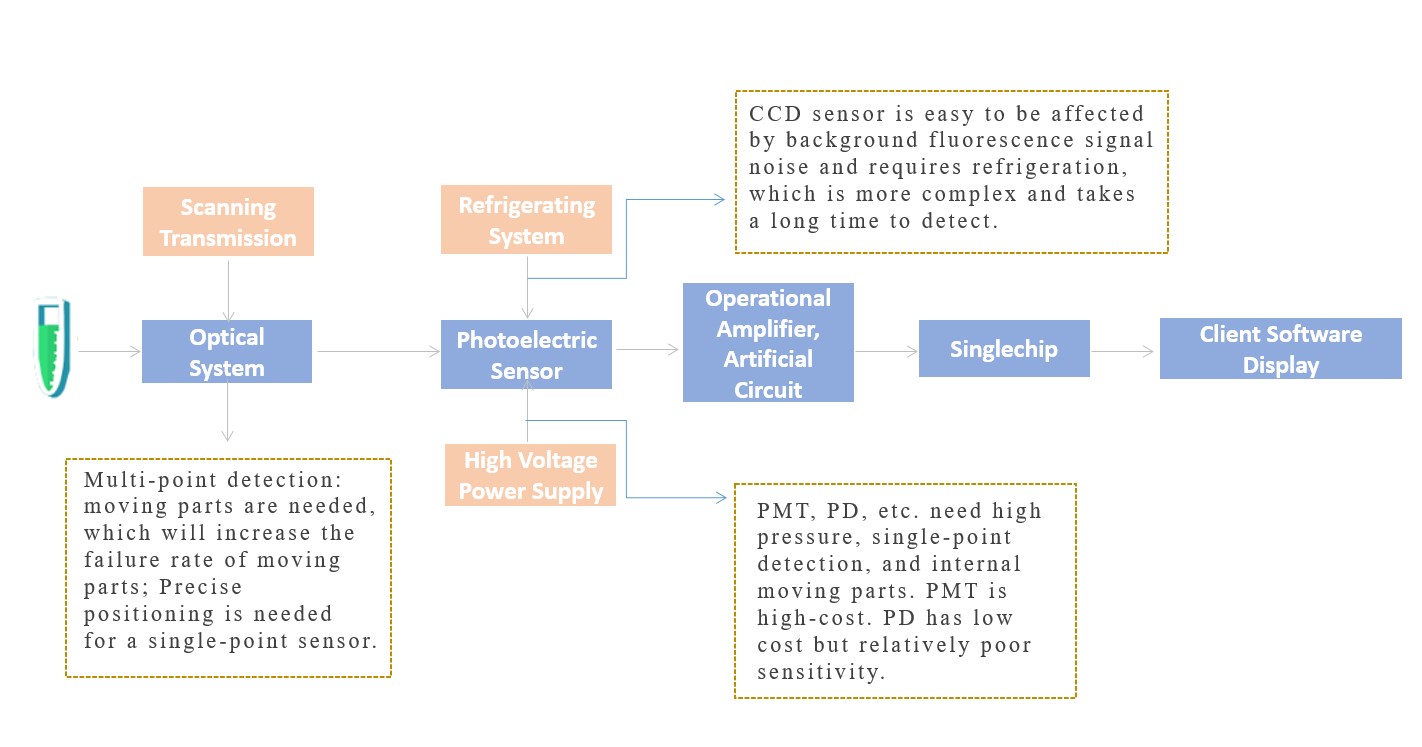
(1) Conventional Technology Platform
Cumbersome, high cost, poor stability, and moving parts require regular calibration. And manufacturers in the industry use outsourcing instead of independent core research and development.
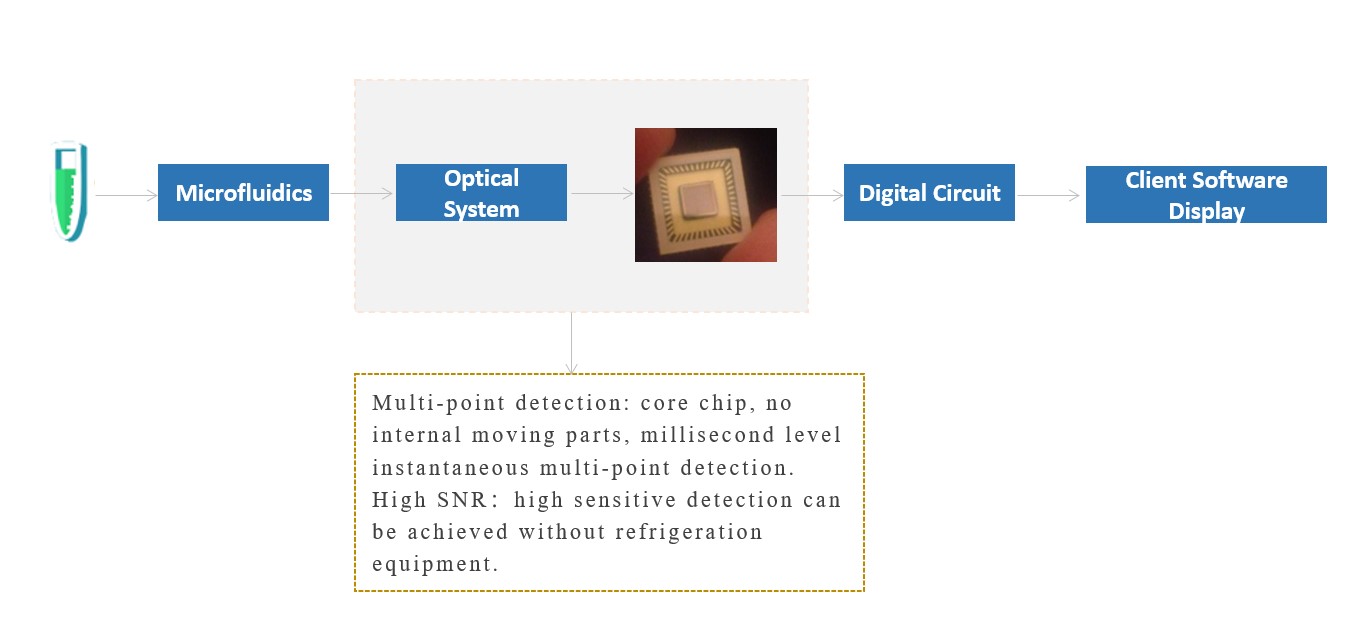
(2) Anitoa's Technology Platform
It has the advantages of high sensitivity, wide dynamic range, high signal-to-noise ratio (no cooling required), low voltage (energy saving), digital circuit (anti-interference), low cost (independent research and development), and no need for regular calibration (maintenance-free).
2.2 Anitoa's Core Competitive Advantages
(1) High photosensitivity: 3.0×10-6 Lux, 1,000 times higher than similar products.
(2) High SNR: "intelligent dark current management" algorithm is adopted;
(3) Can be applied to the qPCR instrument without refrigeration mechanism;
(4) Technical barriers: independent research and development, tape-out, and production;
Professional Portable qPCR System Manufacturer - Anitoa
Anitoa is a professional R&D and manufacturing company of molecular diagnostic qPCR instruments and reagents, with independent intellectual property rights of core chip technology, optical technology, rapid heating, microfluidic control and supporting reagents and other patented technologies, so that the instruments developed by Anitoa are characterized by quick and easy small instruments, allowing the development of expensive and complex large PCR instruments into truly portable POCT products.
Next Article:Viral Nucleic Acid Detection Overall Solution (Part 3)